
Since launching its first Tru-D® Classic device in 2007, Tru-D SmartUVC, a PDI Healthcare environment of care solution, has been committed to having sound science behind our products to demonstrate efficacy.
With launching a new product portfolio, the Tru-D iQ system, in March 2023, we knew we wanted and needed data behind the technology.
“The data for the Tru-D iQ system came from testing in independent labs before the product was approved for commercial use,” said Alice Brewer, MPH, CIC, CPHQ, FAPIC, senior director of clinical affairs for PDI Healthcare. “We had the devices tested in microbiology labs to test efficacy, which is where all of our current data came from.”
In choosing which microorganisms to test against, Tru-D SmartUVC went after the most common epidemiologically important pathogens (EIPs), looking at them from a hierarchy of organism standpoint.1 For instance, if the technology is effective against Clostridioides difficile, which is a microorganism that is difficult to kill, then the technology should be effective against organisms that are more susceptible to disinfection.
For iQ data, Tru-D SmartUVC chose to initially test against C. diff, influenza A (H1N1), Staphylococcus aureus and Candida auris — microorganisms that are prevalent and had also previously been tested against using the Tru-D Classic device.
“The testing on the iQ system was performed under standard laboratory conditions intended to demonstrate the efficacy of the UV dose that is being delivered from the iQ system,” explained Marc-Oliver Wright, MT(ASCP), MS, CIC, FAPIC, clinical science liaison for PDI Healthcare. “We were looking at both UV dose reading and intensity readings as well as log reductions of the selected microorganisms.”
Tru-D SmartUVC was looking for at least a 3 log10 reduction of microorganisms to show efficacy of the technology. In the laboratory clinical data, the iQ system was shown effective against all the microorganisms tested.2
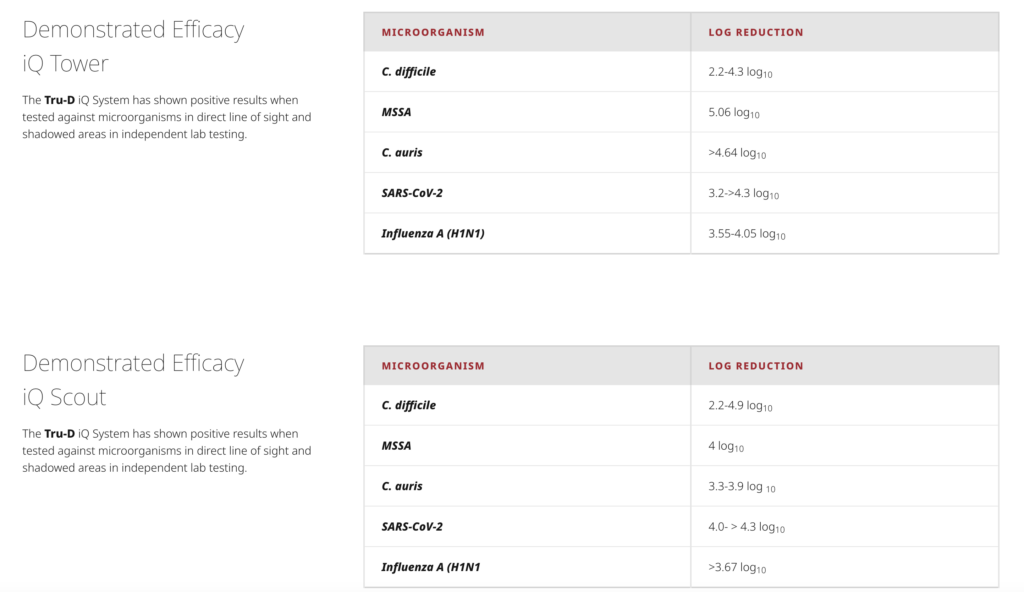
Since the iQ system was not commercially available at the time of the laboratory tests, it was not feasible to conduct real-world testing of the devices.
“The next step is going into the real-world setting,” Brewer said. “Similar to pharmaceutical trials, there are phases of testing, and this phase provided pre-market data. Post-market data will come from real-world testing in a facility setting.”
To learn more, click here.
- https://www.cdc.gov/infectioncontrol/guidelines/disinfection/tables/figure1.htm
-
Testing in independent labs. Data on file.

